

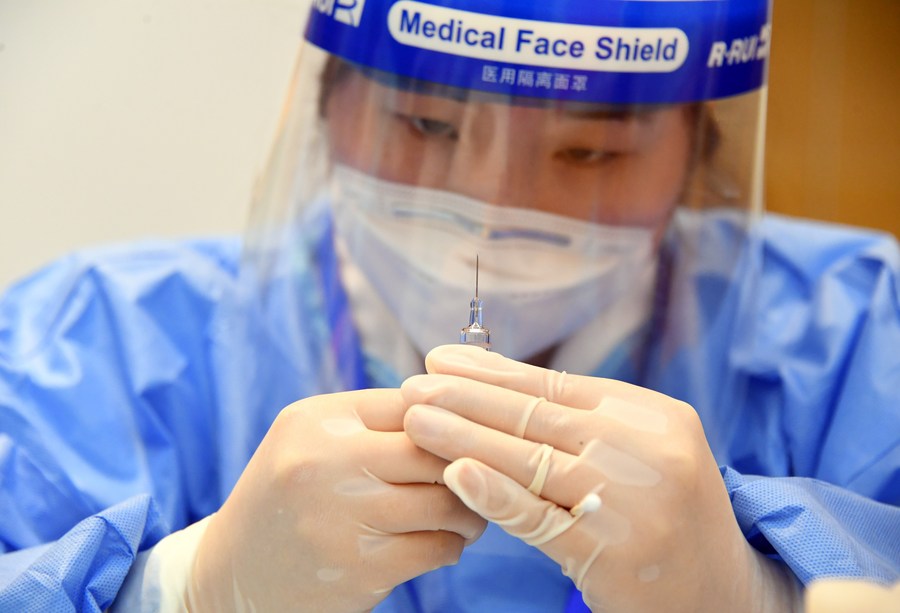
A medical worker prepares a vaccine at a temporary COVID-19 vaccination site at a company in the ZPark Phase 2 in Haidian District of Beijing, capital of China, Feb. 22, 2021. (Xinhua/Ren Chao)
BEIJING, Feb. 26 (Xinhua) -- China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.

 Award-winning photos show poverty reduction achievements in NE China's Jilin province
Award-winning photos show poverty reduction achievements in NE China's Jilin province People dance to greet advent of New Year in Ameiqituo Town, Guizhou
People dance to greet advent of New Year in Ameiqituo Town, Guizhou Fire brigade in Shanghai holds group wedding
Fire brigade in Shanghai holds group wedding Tourists enjoy ice sculptures in Datan Town, north China
Tourists enjoy ice sculptures in Datan Town, north China Sunset scenery of Dayan Pagoda in Xi'an
Sunset scenery of Dayan Pagoda in Xi'an Tourists have fun at scenic spot in Nanlong Town, NW China
Tourists have fun at scenic spot in Nanlong Town, NW China Harbin attracts tourists by making best use of ice in winter
Harbin attracts tourists by making best use of ice in winter In pics: FIS Alpine Ski Women's World Cup Slalom
In pics: FIS Alpine Ski Women's World Cup Slalom Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa
Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa China's FAST telescope will be available to foreign scientists in April
China's FAST telescope will be available to foreign scientists in April