

(file photo)
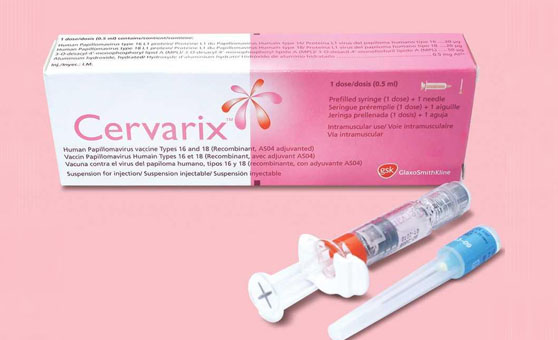
Cervarix, the first approved vaccine for cervical cancer prevention in Chinese mainland, has officially launched, pharmaceutical company GlaxoSmithKline (GSK) announced on July 31, thePaper.cn reported.
The first imported batch of Cervarix has passed inspection by China's quality inspection authority and is heading to the country's community hospitals and health service centers.
Cervical cancer is a common form of malignant tumor that severely threatens the health of women. According to Qiao Youlin, an epidemiology expert at the Chinese Academy of Medical Sciences' Cancer Institute and Hospital, China sees some 100,000 new cases of cervical cancer each year and over 30,000 deaths due to the disease. It is the third most common form of cancer for women between 15 and 44 years of age. "The vaccination combined with screening will significantly reduce the incidence of cervical cancer and pre-cancerous lesions, thus cutting the burden of this disease," said Qiao.
Thomas Willemsen, General Manager of GSK China Pharmaceuticals and Vaccines, noted that the company is undertaking a series of initiatives, including awareness and education campaigns, a complementary platform for adult vaccination, and professional training for healthcare professionals, to protect more Chinese women against cervical cancer.
Cervarix was approved by the China Food and Drug Administration as the first vaccine to prevent cervical cancer in Chinese mainland in July, 2016. Cervical cancer is caused by the oncogenic human papillomavirus (HPV) infection. Studies show that the overall protection of Cervarix against CIN3+ related to any oncogenic HPV types is 93.2 percent.
So far, Cervarix has been registered in 132 countries and regions, and more than 69 million doses of Cervarix have been provided worldwide. Seventy countries have included HPV vaccines in their national immunization programs.
 Fire brigade in Shanghai holds group wedding
Fire brigade in Shanghai holds group wedding Tourists enjoy ice sculptures in Datan Town, north China
Tourists enjoy ice sculptures in Datan Town, north China Sunset scenery of Dayan Pagoda in Xi'an
Sunset scenery of Dayan Pagoda in Xi'an Tourists have fun at scenic spot in Nanlong Town, NW China
Tourists have fun at scenic spot in Nanlong Town, NW China Harbin attracts tourists by making best use of ice in winter
Harbin attracts tourists by making best use of ice in winter In pics: FIS Alpine Ski Women's World Cup Slalom
In pics: FIS Alpine Ski Women's World Cup Slalom Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa
Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa China's FAST telescope will be available to foreign scientists in April
China's FAST telescope will be available to foreign scientists in April "She power" plays indispensable role in poverty alleviation
"She power" plays indispensable role in poverty alleviation Top 10 world news events of People's Daily in 2020
Top 10 world news events of People's Daily in 2020 Top 10 China news events of People's Daily in 2020
Top 10 China news events of People's Daily in 2020 Top 10 media buzzwords of 2020
Top 10 media buzzwords of 2020 Year-ender:10 major tourism stories of 2020
Year-ender:10 major tourism stories of 2020 No interference in Venezuelan issues
No interference in Venezuelan issues
 Biz prepares for trade spat
Biz prepares for trade spat
 Broadcasting Continent
Broadcasting Continent Australia wins Chinese CEOs as US loses
Australia wins Chinese CEOs as US loses