

Xinhua Poto
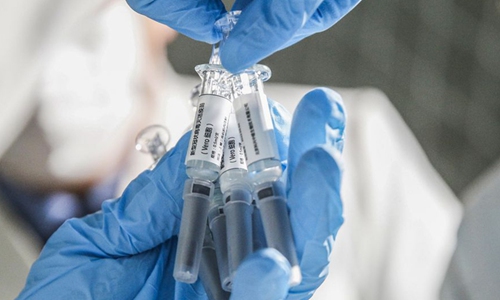
Six COVID-19 vaccine candidates, including three from China, have entered phase-3 trials, a senior World Health Organization (WHO) official said on Thursday.
The three Chinese candidates are from Sinovac, Wuhan Institute of Biological Products/Sinopharm and Beijing Institute of Biological Products/Sinopharm, said Michael Ryan, executive director of the WHO Health Emergencies Program, at a virtual briefing.
The other three are made by the University of Oxford/AstraZeneca, Moderna/NIAID and BioNTech/Fosun Pharma/Pfizer, he added.
The vaccines will be put into the general population for the first time in phase 3, after previous trials have focused on safety, immunogenicity and immune response in a small number of humans, said the WHO official. The phase-3 trial will test whether the vaccines can "protect large numbers of people over a prolonged period of time."
However, "phase-3 doesn't mean nearly there," he said, as "there is no guarantee that any of these six will give us the answer."
In total, 165 vaccine candidates have started some forms of trials, and 26 of them in clinical trials, according to WHO records.
 Fire brigade in Shanghai holds group wedding
Fire brigade in Shanghai holds group wedding Tourists enjoy ice sculptures in Datan Town, north China
Tourists enjoy ice sculptures in Datan Town, north China Sunset scenery of Dayan Pagoda in Xi'an
Sunset scenery of Dayan Pagoda in Xi'an Tourists have fun at scenic spot in Nanlong Town, NW China
Tourists have fun at scenic spot in Nanlong Town, NW China Harbin attracts tourists by making best use of ice in winter
Harbin attracts tourists by making best use of ice in winter In pics: FIS Alpine Ski Women's World Cup Slalom
In pics: FIS Alpine Ski Women's World Cup Slalom Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa
Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa China's FAST telescope will be available to foreign scientists in April
China's FAST telescope will be available to foreign scientists in April "She power" plays indispensable role in poverty alleviation
"She power" plays indispensable role in poverty alleviation Top 10 world news events of People's Daily in 2020
Top 10 world news events of People's Daily in 2020 Top 10 China news events of People's Daily in 2020
Top 10 China news events of People's Daily in 2020 Top 10 media buzzwords of 2020
Top 10 media buzzwords of 2020 Year-ender:10 major tourism stories of 2020
Year-ender:10 major tourism stories of 2020 No interference in Venezuelan issues
No interference in Venezuelan issues
 Biz prepares for trade spat
Biz prepares for trade spat
 Broadcasting Continent
Broadcasting Continent Australia wins Chinese CEOs as US loses
Australia wins Chinese CEOs as US loses